Base Oil Classification
MINERAL OIL
Modern refining technology technicians has made it possible to produce lubricants of good quality from a wide variety of crude oils. Refining crude oil is the process of separating the crude oil into different fractions or cuts. These cuts are called naphtha, gasoline, kerosene, light and heavy oils and residues. Each type of crude oil gives different amount of each ‘cut’. Basically crude oils are of two types namely paraffin and naphthalene.
MINERAL OIL PLUS ADDITIVE
A refinery makes only the base lube oil stocks of different viscosity. They are unsuitable for direct consumption. Therefore, oils are mixed to attain right viscosity and additives are added to improve other qualities.
SYNTHETIC LIQUID LUBRICANTS
Synthetic liquid lubricants can be characterized as oily and neutral liquids. They are not obtained from petroleum crude oils. But they have almost similar properties as petroleum lubricants. These find application in situations where petroleum oils cannot be used. Some specific chemical classes of synthetic lubricants are Di-esters, organic-phosphate esters, silicone polymers etc. Approximately 95 percent of the current lubricant market share is comprised of conventional (mineral-based) oils. Most people know these mineral oils are derived from a crude stock, but how much do you really know about the refining process?
LUB REIFINARY
The petroleum that flows from a well in the form of crude oil comes in many varieties and types, ranging from light-colored oils containing mostly small hydrocarbon molecular chains to black, nearly solid asphalt-like large hydrocarbon chains. These crude oils are very complex mixtures containing a plethora of different compounds made of hydrogen and carbon. These compounds (known as hydrocarbons) can range in size from methane (containing one carbon and four hydrogen atoms) to massive structures with 60 or more carbon atoms. This molecular size distribution can be used to our advantage. Most lubricating oils come from petroleum or crude oil. In order to get lubricating oil from
a crude oil, the crude oil must be sent through a refinery. The refinery takes from the crude oil a lot of molecules of various sizes and structures that can be used for different things. For example, gasoline, diesel and kerosene are all derived from crude oil. Lubricating oil relates to hydrocarbon molecules of a particular size (in the range from 26 to 40 carbons). Fairly large and heavy molecules are needed to work as lubricating oils. The molecules that are used with gasoline and kerosene are smaller and have fewer carbons in the structure of the molecule. The refinery puts these molecules in little silos based on size and weight, and removes impurities, enabling each of the products from the crude oil to be utilized. After the crude oil is desalted and sent through a furnace where it is heated and partially vaporized, it is sent to a fractional column. This column operates slightly above atmospheric pressure and separates the hydrocarbons based on their boiling points, which are directly affected by their molecular size. In the fractional column, heat is applied and concentrated at the bottom. The hydrocarbons entering the column will be vaporized. As they
travel upward in the column, they will cool until they condense back into a liquid form. The point at which this condensation occurs varies again based in part on the molecular size. By pulling the condensing liquid from the column at different heights, you can essentially separate the crude oil based on molecular size. The smallest of the hydrocarbons (5 to 10 carbon atoms) will rise to the very top of the column. They will be processed into products
like gasoline. Condensing just before reaching the top, the compounds containing 11 to 13 carbon atoms will be processed into kerosene and jet fuel. Larger still at 14 to 25 carbon atoms in the molecular chain, diesel and gas oils are pulled out. Those compounds with 26 to 40 carbon atoms are a tribologist’s main concern. This is the material used for the creation of lubricating oil. At the bottom of the column, the heaviest and largest of the hydrocarbons (40-plus carbon atoms) are taken and used in asphalticbased products. After the distillation process, the compounds need to be refined for their intended purpose. This step in the process is done to reduce the tendency of the base oil to age (oxidize) in service and also to improve the viscosity/temperature characteristics. There are two ways this can be done. The first involves a separation process where there are two products
being made: a desired lube product and undesirable byproducts. The second way, which is quickly becoming the favored of the two, is a conversion process. This process involves converting undesirable molecular structures into desirable structures with the use ofhydrogen, heat and pressure.
EXTRACTION PROCESS
The following is a simplified description of the extraction process:
1-DISTILLATION ــــــDEASPHALTINGـــــــSOLVENT EXTRACTIONـــــــFINISHINGـــــــFINAL BASE OIL
DE-ASPHALTING
Propane de-asphalting takes the residuum from the very bottom of the column (the heaviest, largest molecules) and separates them into two products: tar and compounds that are similar to the lube distillates but have a higher boiling point. This material is called de-asphalted oil, and it will be refined in the same manner as the lube distillates.
SOLVENT EXTRACTION
Solvent extraction is the term used for the removal of most of the aromatics and undesirable constituents of oil distillates by liquid extraction. Commonly used solvents contain phenol, furfural and sulphur dioxide. The resulting base stocks are raffinates (referred to as neutral oils) and an extract that is rich in aromatic content, which is highly sought after as a process oil or fuel oil.
DE-WAXING
After solvent extraction, the raffinates are dewaxed to improve low-temperature fluidity. This process again produces two products: a byproduct wax that is almost completely paraffinic and dewaxed oil that contains paraffins, naphthenic and some aromatics. This dewaxed oil becomes the base stock for many lubricants, but there is one more process that can be done to make a premium product.
HYDROFINISHING
Hydro finishing changes the polar compounds in the oil by a chemical reaction involving hydrogen. After this process, an observer would notice a lighter-colored product and an improved chemical stability. The final quality of the base oil is determined by the severity of the application of temperature and pressure in the hydro finishing process.
CONVERSION PROCESS
The following is a simplified description of the conversion process:
1-DISTILLATION ــــــHYDROCRACKINGـــــــHYDRODEWAXINGـــــــHYDROTREATINGـــــــFINAL BASE OIL
HYDROCRACKING
In this refining process, the distillates are subjected to a chemical reaction with hydrogen in the presence of a catalyst at high temperatures and pressures (420 degrees C and 3,000 psi). The aromatic and naphthene rings are broken, opened and joined using hydrogen to form an isoparaffin structure. The reaction with hydrogen will also aid in the removal of water, ammonia and hydrogen sulfide.
HYDRODEWAXING
During hydrodewaxing, much like hydro cracking, a hydrogenation unit is used to deploy a catalyst that is specific to conveying waxy normal paraffins to more desirable isoparaffin structures.
HYDROTREATING
Because the previous two processes involve breaking chemical bonds between two carbon atoms, it is necessary to introduce the saturation of any unsaturated molecules. This is easily done by introducing more hydrogen. These saturated molecules are more stable and will be able to resist the oxidation process better than the unsaturated variety.
There are slight differences in the characteristics of the finished base oil produced by these two processes. The main difference lies in the aromatic content. The conversion process can reduce the aromatic content to around 0.5 percent, while the extraction process lingers around 15 to 20 percent. This aromatic content has the following effects:
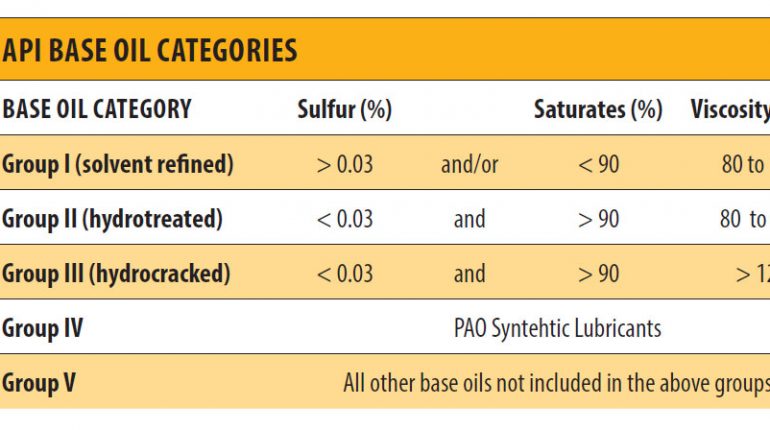
It would appear that the conversion process produces a better quality product, but there is always a trade-off. The cost of refining oil using the conversion process is somewhat higher than the extraction process. This extra cost incurred by the refiner is eventually passed on to the customer. However, in this case, the customer typically gets what he pays for higher quality base oil at a higher initial price. Almost every lubricant used in plants today started off as just base oil. The American Petroleum Institute (API) has categorized base oils into five categories (API 1509, Appendix E). The first three groups are refined from petroleum crude oil. Group IV base oils are full synthetic (polyalphaolefin) oils. Group V is for all other base oils not included in Groups I through IV. Before all the additives are added to the mixture, lubricating oils begin as one or more of these five API groups.
GROUP I
Group I base oils are classified as less than 90 percent saturates, greater than 0.03 percent sulfur and with a viscosity-index range of 80 to 120. The temperature range for these oils is from 32 to 150 degrees F. Group I base oils are solvent-refined, which is a simpler refining process. This is why they are the cheapest base oils on the market.
GROUP II
Group II base oils are defined as being more than 90 percent saturates less than 0.03 percent sulfur and with a viscosity index of 80 to 120. They are often manufactured by hydro cracking, which is a more complex process than what is used for Group I base oils. Since the entire hydrocarbon molecules of these oils are saturated, Group II base oils have better antioxidation properties. They also have a clearer color and cost more in comparison to Group I base oils. Still, Group II base oils are becoming very common on the market today and are priced very close to Group I oils.
GROUP III
Group III base oils are greater than 90 percent saturates, less than 0.03 percent sulfur and have a viscosity index above 120. These oils are refined even more than Group II base oils and generally are severely hydro cracked (higher pressure and heat). This longer process is designed to achieve purer base oil. Although made from crude oil, Group III base oils are sometimes described as synthesized hydrocarbons. Like Group II base oils, these oils are also becoming more prevalent.
GROUP IV
Group IV base oils are polyalphaolefin (PAOs). These synthetic base oils are made through a process called synthesizing. They have a much broader temperature range and are great for use in extreme cold conditions and high heat applications.
GROUP V
Group V base oils are classified as all other base oils, including silicone, phosphate ester, polyalkylene glycol (PAG), polyolester, biolubes, etc. These base oils are at times mixed with other base stocks to enhance the oil’s properties. An example would be PAO-based compressor oil that is mixed with a polyolester. Esters are common Group V base oils used in different lubricant formulations to improve the properties of the existing base oil. Ester
oils can take more abuse at higher temperatures and will provide superior detergency compared to PAO synthetic base oil, which in turn increases the hours of use. Remember, whichever base oil you choose, just be sure it is appropriate for the application, temperature range and conditions in your plant.
THE CHANGING USE OF BASE OILS
A recent study on the use of base oils in today’s plants in comparison to a little more than a decade ago found a dramatic change has occurred. Present-day Group II base oils are the most commonly used base oils in plants, making up 47 percent of the capacity of plants in which the study was conducted. This compared to 21 percent for both Group II and III base oils just a decade ago. Currently, Group III accounts for less than 1 percent of the capacity in plants. Group I base oils previously made up 56 percent of the capacity, compared to 28 percent of the capacity in today’s plants.

